BioPharma
Solutions
Localizing Precision
NGS Testing, Globally
At Pillar Biosciences, we understand that biopharmaceutical companies require testing partners who maintain the highest standards of quality and regulatory compliance. We operate under strict design control, following a meticulous assay development process while adhering to comprehensive guidelines that ensure quality, reducing risk for our partner companies.
Our processes ensure consistent assay kit development and reliable NGS results that meet the strict requirements of pharmaceutical research and development.
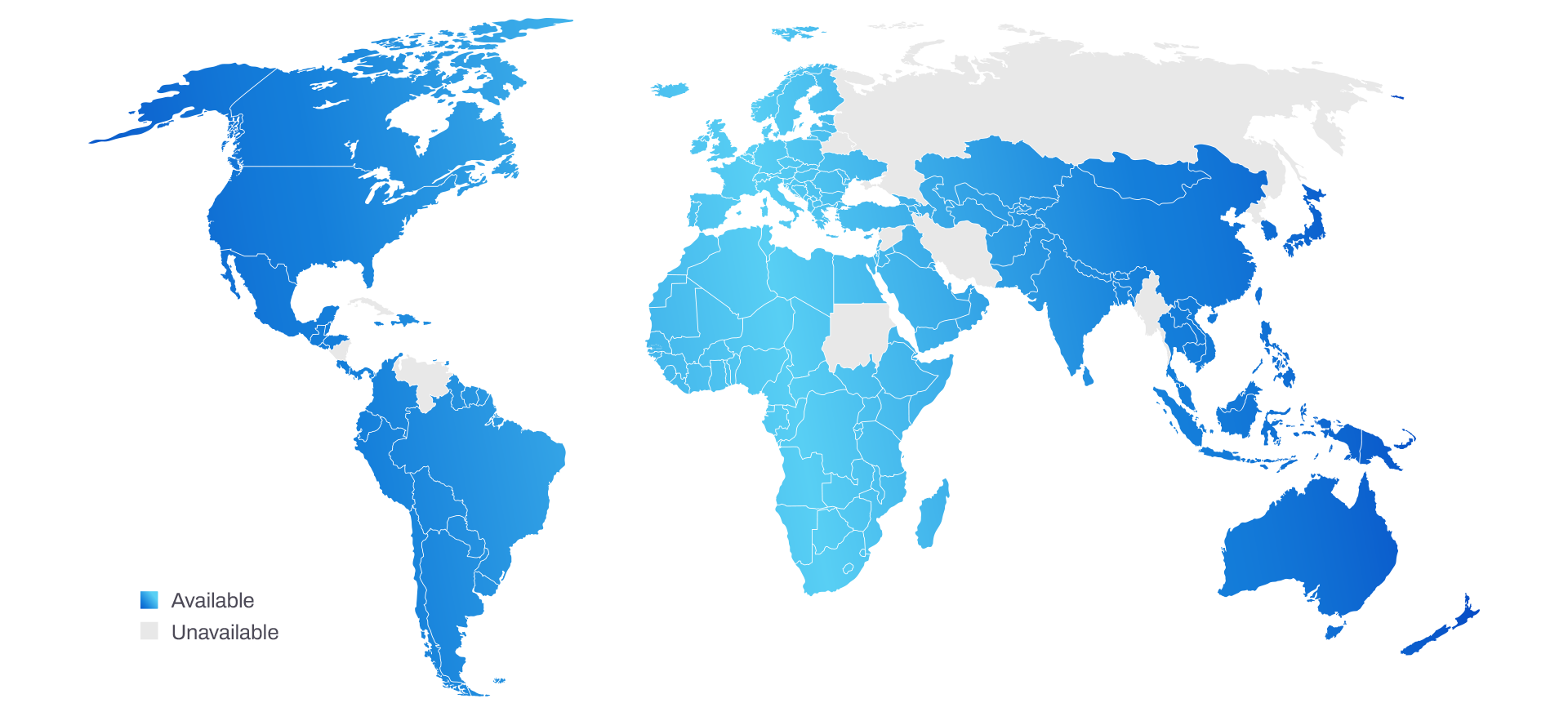
Pillar’s Global Reach
With a presence in over 140 countries and more than ~300 clinical accounts worldwide, Pillar’s solutions are trusted by leading institutions across the globe. Our global infrastructure guarantees reliable support and logistics wherever your program needs to go.

Established Reputation with Premier Institutional Partners
Pillar has built strong collaborative relationships with top-ranked institutions and high-volume national laboratories across the pharmaceutical and clinical research landscape.
Our technologies have been validated through rigorous testing and implementation at leading national reference laboratories, leading cancer centers, academic medical institutions, and pharmaceutical companies.
Cost-Effective Kitted NGS Solutions Without Compromising Quality
Our rapid, targeted NGS panels provide a more economical solution while delivering the focused, actionable clinical value you need. Our targeted NGS panels offer significant advantages:
- Focused content that reduces unnecessary sequencing and costs
- Streamlined workflows that minimize resource utilization
- Higher sample throughput capabilities on low to mid-throughput NGS systems that improve operational efficiency
By concentrating on clinically relevant content and ensuring your genes of interest are covered, we deliver precisely what you need without the expense of running complex NGS workflows.
Commercialization Capabilities Across Key Global Markets
Through our strategic alliance with Illumina, Pillar brings global market access and clinical support in the US, Europe, Japan, China, Latin America and beyond. We drive early market engagement, establishing access sites and building commercial momentum prior to drug approval, ensuring your therapies reach patients faster and more efficiently.

Global Regulatory Expertise That Inspires Confidence
Pillar Biosciences brings deep regulatory experience to every pharma partnership. Our team has successfully navigated FDA and CE (Europe) approvals and offers specialized support for clinical testing, submissions and approvals in China. Our expertise helps ensure your diagnostic program is positioned for global regulatory success, with proven strategies for streamlining submissions and accelerating approvals in major markets.
Pillar Biosciences has Successfully Obtained:
- FDA PMA IVD / CDx approval
- CE IVD / IVDR marking for European market access
- China NMPA approvals through our strategic partnerships
- Compliance with FDA 21 CFR Part 820 and successful
FDA inspection
This regulatory experience gives our pharmaceutical partners
confidence that Pillar understands the compliance requirements
critical to bringing diagnostic products to market globally.
Clinical Research Capabilities
Our partnerships extend to the clinical trial space, where we work with Global CROs to perform clinical trial testing. For pharmaceutical companies developing companion diagnostics, this means:
- Pillar CLIA/CAP certified laboratory services for project-based feasibility testing
- Proof-of-concept validation work to support your research goals
- Small-scale project capabilities that allow for nimble adaptation to your specific needs

Flexible Biopharma Collaboration
Pillar prides itself on being flexible in our approach to our biopharma partnerships. We develop customized technical agreements that address your specific business requirements, adapting our solutions to help meet your evolving global needs.
Contact our team today to discuss how Pillar Biosciences can support your pharmaceutical development programs with our precision NGS solutions.
Why Partner with Pillar Biosciences?

Regulatory expertise in all major markets, including China

Peer-reviewed scientific validation and real-world impact

Proven commercialization strategies and global market access
Dedicated to early engagement and collaborative program development

Transparent, active install base and clinical site engagement
Strategic Partnerships
Supporting Your Program
Pillar maintains strategic relationships with industry leaders, including Illumina, enhancing our ability to deliver comprehensive solutions to our pharmaceutical partners.

Want to be the first to know about new and exciting news at Pillar Biosciences?
Sign up for our mailing list here
