oncoReveal® CDx
Pan-Cancer Solid Tumor IVD
Indications for Use
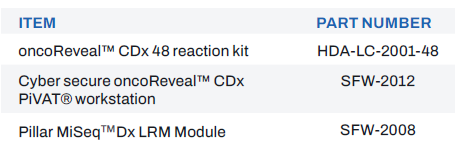
The oncoReveal® CDx is a qualitative next generation sequencing based in vitro diagnostic test that uses amplicon-based target enrichment technology for detection of single nucleotide variants (SNVs), insertions and deletions in 22 genes using DNA isolated from formalin-fixed paraffin-embedded (FFPE) tumor tissue specimens and using the Illumina MiSeq™Dx. The test is intended as a companion diagnostic to identify patients who may benefit from treatment with the targeted therapies listed in Table 1 in accordance with the approved therapeutic product labeling.
Additionally, oncoReveal® CDx is intended to provide tumor mutation profiling to be used by qualified health care professionals in accordance with professional guidelines in oncology for previously diagnosed cancer patients with solid malignant neoplasms. Genomic findings other than those listed in Table 1 are not prescriptive or conclusive for labeled use of any specific therapeutic product.
Table 1: Approved Companion Diagnostic and Tumor-Profiling Indications
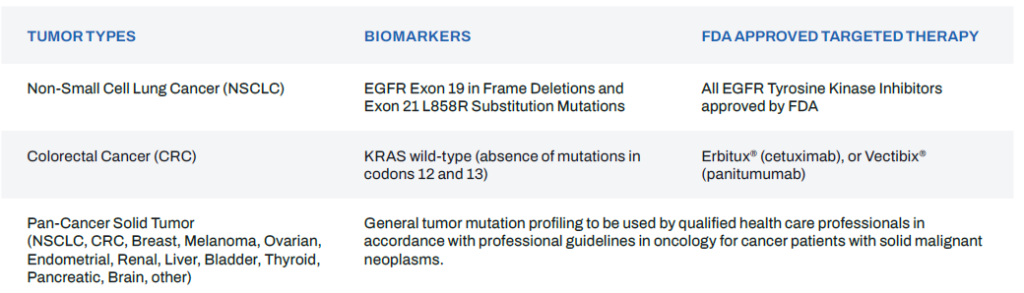
Table 2: oncoReveal® CDx gene list*
oncoReveal® CDx Technical Specifications

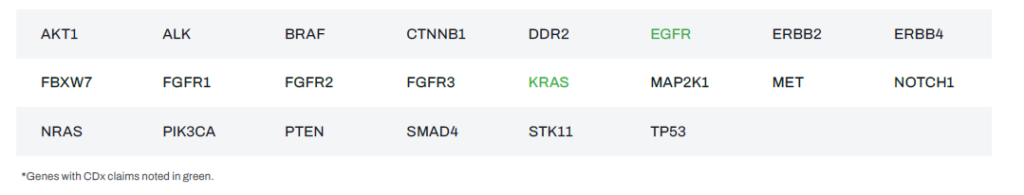
Ordering Information